It’s been quite a year for the Pharma Industry. Although there weren’t any massive surprises, the industry was on a rollercoaster ride backed by some key M&As, outbreaks, and pathbreaking research.
Evaluate Group has come up with its predictions for the upcoming year and what it may hold. In a study titled, EP Vantage 2017 Preview, the study made some substantial quantitative forecasts. Here are the highlights-
- Pfizer to dominate prescription and OTC sales in 2017, banking nearly $50 billion
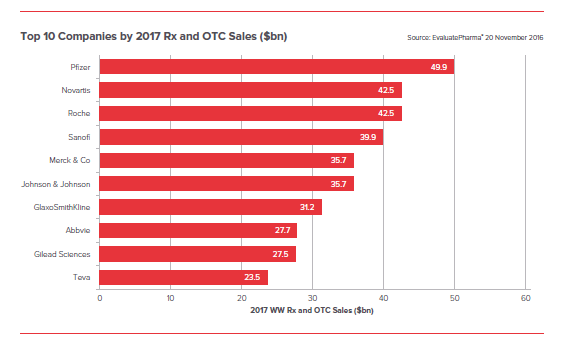
Pfizer maintained its pole position through acquisitions of Hospira for $17bn and Medivation for $14bn, so further substantial moves by the pharma giant cannot be ruled out next year.
Since Sanofi missed out on Medivation which fell to Pfizer in September 2016, its acquisitive ambitions cannot be neglected.
And Johnson & Johnson, quite recently, confirmed its interest in Switzerland’s Actelion – this target would provide immediate growth for any buyer, although it would come with a huge price tag.
- Humira remaining the industry’s biggest selling drug
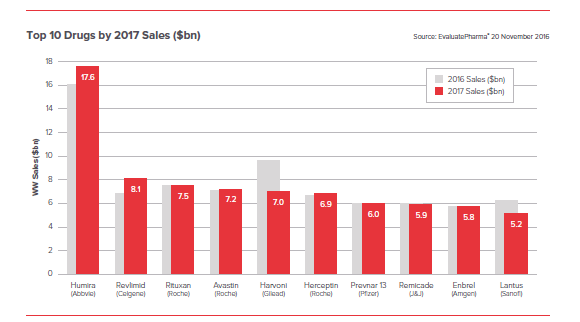
It is notable that only Humira and Revlimid are showing growth among the top 10. The world’s biggest blockbusters are obviously not going to be in explosive growth stage, but this analysis suggests that the next few years will see a change in guard at the top, thanks to biosimilars making inroads.
- Progress in early-stage investigations of cutting-edge scientific techniques - CAR-T, Crispr gene editing and gene therapies
A U.S. clinical trial using CRISPR-edited genes to treat various cancers is due to start in early 2017. And early next year, Beijing University scientists hope to begin three clinical trials using gene-editing to fight bladder, prostate and renal-cell cancers.
One of the most important elements of CRISPR development in China is scale. It's being deployed in many different ways, in many different labs.
ANALYSIS & PREDICTIONS
1. Top Companies by New Sales
Looking at the companies’ forecast to add the most, new sales next year, an entirely different ranking emerges.
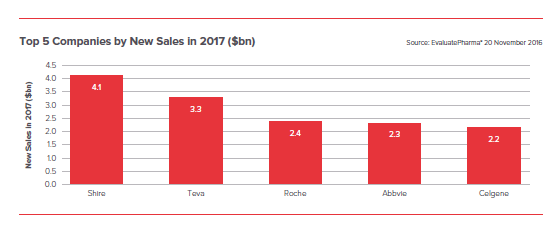
Shire acquired Baxalta and Teva consolidated Allergan’s generics business, and thus, became the leaders
Abbvie and Celgene will survive on Humira and Revlimid respectively, which astonishingly rank as two of the biggest-growing products next year. Having been launched in 2003 and 2006 respectively these brands are still adding more than $1bn in sales a year.
2. The Expected Upgrades and Downgrades
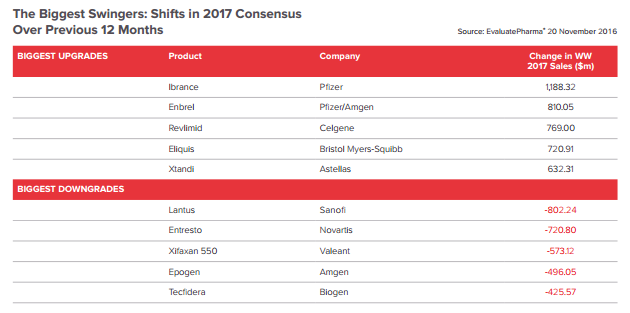
This year Sanofi’s insulin Lantus was removed from a number of formularies in favour of Lilly’s approaching biosimilar Basaglar; as a result almost a billion dollars was erased from Sanofi’s 2017 sales forecast over the course of 2016. On the flip side, Enbrel has seen substantial upgrades. This analysis was conducted before Amgen’s third-quarter warning, serving to demonstrate that many analysts are still underestimating the extent of the on-going price battle in the US.
CONCLUSION
For many years industry has relied on the often-quoted and often-disputed statistic of a billion dollars to develop a drug. Given the sophisticated analysis of drug pricing that many are now accustomed to, this statistic is too simplistic to keep returning to. And it certainly does not justify the sorts of aggressive price hikes that have tarnished the whole sector.
Of course, that billion-dollar number was also supposed to include drug failures, further examples of which 2017 will no doubt provide. It also encompassed regulatory setbacks, which seemed fairly common in 2016.
So while those whose businesses involve striking deals are perhaps predictably positive for the prospects of 2017, this preview of the coming year will end on a statistic on R&D productivity. At the end of the day, there is really only one way to judge the performance of the drug development sector: by looking at its output.
Of course, this is in itself a controversial and complex aspect to measure and the analysis below, looking at FDA approvals of novel molecules, is far from perfect. For example, it does not capture the breadth of a drug that has utility in many settings – although the inclusion of fifth-year sales forecasts attempts to signal this – and it provides no indication of input, in either time or money, to generate these drugs.
These caveats aside, it is clear that 2016 was a pretty woeful year for new drug approvals. With only one month of the year left, it looks like only 25 novel biologics or small molecules will have passed regulatory muster in the US. After a couple of years of scorching drug approval rates and huge fifth-year sales estimates, this is a disappointing dip in productivity.
Information Source: https://info.evaluategroup.com/rs/607-YGS-364/images/EPV2017Prev.pdf
For More interesting reads go to: Scientific Animations Blog Page








