Influenza or simply “the flu” is a common infectious disease caused by influenza viruses. It is usually characterized by high fever, runny nose, sore throat, and body ache / headache.
Influenza is a highly contagious, airborne disease that occurs in seasonal epidemics and may manifest as an acute illness with symptoms ranging from mild fatigue to respiratory failure and even death. Every year, influenza consumes about 250,000 to 500,000 lives, and leaves 3-5 million severely ill. The outbreaks usually spike during winter but can happen at any time.
Pathophysiology
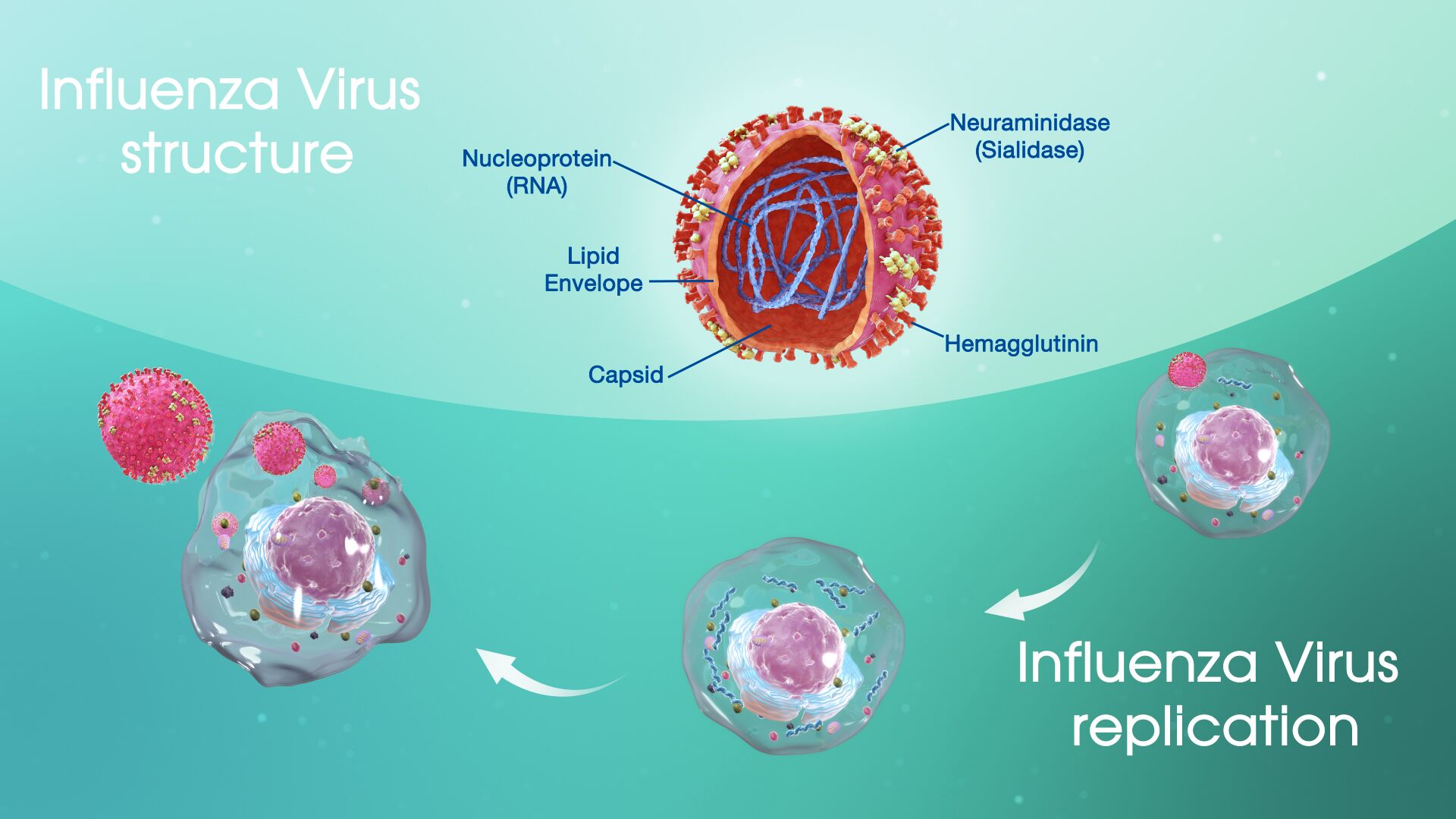
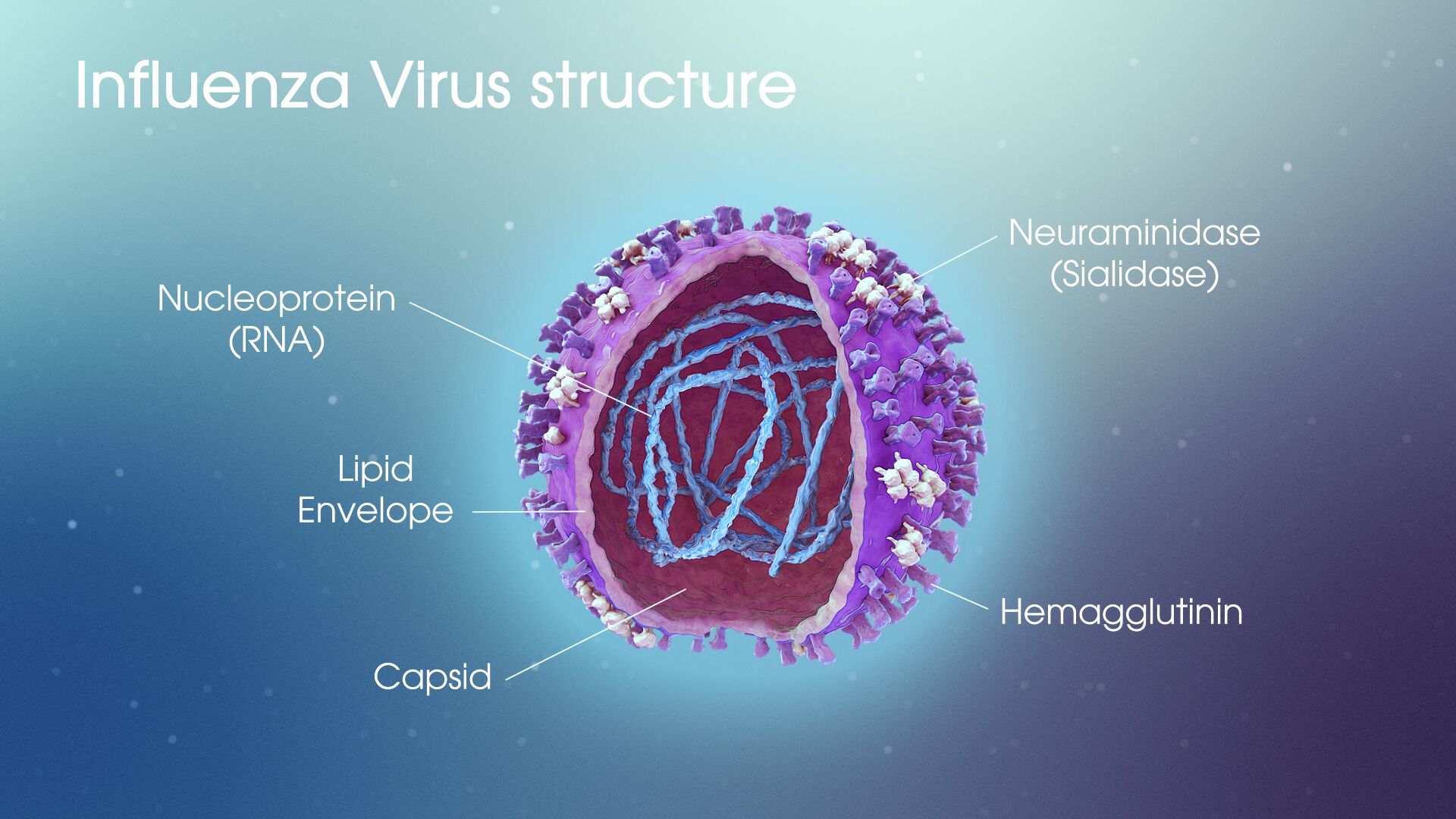 Structure of the influenza virus
Structure of the influenza virus
The flu virus is an enveloped, single-stranded RNA virus. The core nucleoproteins are used to distinguish the 3 types of influenza viruses: A, B, and C. Influenza A causes most human and all avian influenza infections.
The RNA core consists of 8 gene segments surrounded by a coat of 10 (influenza A) or 11 (influenza B) proteins. Immunologically, the most significant surface proteins include hemagglutinin (H) and neuraminidase (N). Subclassification of influenza A occurs on the basis of H and N proteins.
Transmission and infection
The flu transmits most commonly through air, when an infected person ejects droplets while coughing or sneezing. Contaminated surfaces can also spread the virus.
The main targets of the influenza virus are the columnar epithelial cells of the respiratory tract. If these cells bear functional viral receptors, they automatically become susceptible to infection. Certain areas of the binding site are highly conserved between subtypes of the influenza virus.
Meanwhile, the host cell may resist the viral attachment by several mechanisms:
- Secretion of specific IgA antibodies,
- Production of mucoproteins that able to bind to virus (H protein), and
- Genetic diversification of the host receptor
Once the cell membrane and the virus have been closely juxtaposed by virus-receptor interaction, the complex is endocytosed.
The viral H protein undergoes a conformational rearrangement in the acidic cell environment, bringing the viral and endosomal membranes close enough to fuse. To allow release of viral RNA into the cytoplasm, the H+ ions in the acidic endosome are pumped into the virion interior by the M2 ion channel. Cellular proteases (protein-digesting enzymes) are often required to cleave viral proteins to form the mature infectious virus particle. These enzymes cleave the polypeptide hemagglutinin chain precursor of extracellular particles that activates H protein in virions and renders them infectious.
Replication occurs within hours and numerous virions are produced leading to cellular dysfunction and degeneration. Infectious particles bud out from the apical plasma membrane of epithelial cells into the airways. This favors the swift spread of the virus within the lungs due to the rapid infection of neighboring cells.
The usual incubation periods of influenza range from 1 to 4 days. Therefore, it may be possible for transmission to occur via asymptomatic persons who are unaware of their own infection.
Role of H and N proteinsBinding of the influenza virus and its cleavage from the binding site at the host cell are two major events of viral infection that are regulated by H and N proteins. Hemagglutinin or H protein determines which species a viral strain can infect as well as decides where in the human respiratory tract the flu virus would bind. Neuraminidase or N protein cleaves the bond that holds newly replicated virions to the cell surface, letting the infection to spread.
Avirulent (non-virulent) strains have a hemagglutinin structure that can only be cleaved by the proteases of throat and lungs, so they can not infect other tissues. In humans, the replication of the influenza virus is generally restricted to the epithelial cells of the upper and lower respiratory tract.
Some highly virulent avian influenza strains, however, contain genetic insertions at the cleavage site of hemagglutinin leading to processing by all proteases. This lets the infection spread throughout the body.
Virulence of the influenza virus depends on the compatibility of neuraminidase with hemagglutinin. A virulent virus which has undergone mutations in the hemagglutinin needs compensatory mutations in the neuraminidase to maintain its virulence.
Since hemagglutinin and neuraminidase are critical for virulence, they are major targets for the neutralizing antibodies to influenza (acquired immunity).
Treatment modalities
- Prevention is the most effective step to combat flu. WHO recommends yearly vaccinations for high risk people. The vaccine is usually well-tolerated and effective against 3-4 types of influenza, but needs to be upgraded yearly as the virus evolves rapidly.
- Doctors usually advise flu patients to drink plenty of fluids and take rest. Acetaminophen or paracetamol is recommended to relieve the fever and muscle pain. Aspirin intake must be avoided during the infection as it adversely affects the liver and may cause Reye’s syndrome in children and teenagers.
- Antiviral drugs can be effective within the first 48 hours to the appearance of symptoms. Children, elderly and pregnant women have their immune system compromised and, hence, carry higher risk.
The two classes of antiviral drugs used against influenza are neuraminidase inhibitors (oseltamivir, zanamivir, laninamivir and peramivir) and M2 protein inhibitors (adamantane derivatives).
- Neuraminidase inhibitors
In flu patients, neuraminidase inhibitors are observed to reduce the length of time the symptoms show up by slightly less than a day. However, they did not appear to cut down the risk of complications such as pneumonia. Rising resistance to neuraminidase inhibitors has led researchers to seek alternative antiviral drugs with different mechanisms of action.
- M2 inhibitors
Amantadine and rimantadine inhibit the viral ion channel for the M2 protein, thereby preventing the influenza A virus replication. These drugs are often effective against influenza A in the early phase of infection. But they cannot inhibit influenza B viruses from replicating as they lack the M2 drug target.
Since 2006, use of M2 inhibitors has greatly reduced because of widespread resistance to the adamantanes among influenza A (H3N2) virus strains. Oseltamivir resistance has also emerged in the US during the 2008-2009 influenza season.
Research trends
Innovative and continuous research is needed in order to better understand viral pathogenesis and genomics, as well as the body's immune system response to the infection to aid the development of antiviral drugs and vaccines for influenza.
A research program called Influenza Genome Sequencing Project is creating a library of influenza sequences. The library gives a clear picture of how the virus evolves over time and also helps to identify the factors (genes) that affect immunogenicity and make one strain more lethal than another.
Despite advances in the field, most vaccine formulations for influenza are still produced by rather old-fashioned techniques that have been in use for over 60 years. Such methods involve the growth and passaging of the vaccine strains in embryonated chicken eggs, therefore production and subsequent formulation can take several months and rely upon the availability of the eggs.
Increasing demands for influenza vaccines, especially during pandemic, necessitates new research approaches to develop egg-independent manufacturing systems. The first egg-free recombinant influenza vaccine (produced in an insect cell line) was approved by the US FDA in January 2013 and was available for the influenza season of 2013-2014, demonstrating the proof of principle.
The sequencing of the influenza genome and recombinant DNA technology may accelerate the generation of new vaccine strains by allowing scientists to substitute new antigens into a previously developed vaccine strain. New technologies are also being developed to grow viruses in cell culture, which promise higher yields, less cost, better quality and surge capacity.
Research on a universal influenza A vaccine, targeted against the external domain of the transmembrane viral M2 protein, is being done at the University of Ghent by Walter Fiers, Xavier Saelens and their team and has now successfully concluded Phase I clinical trials. There has been some research success towards a "universal flu vaccine" that produces antibodies against proteins on the viral coat which mutate less rapidly, and thus a single shot could potentially provide longer-lasting protection.
A number of biologics, therapeutic vaccines and immunobiologics are also being investigated for treatment of infection caused by viruses. Therapeutic biologics are designed to activate the immune response to virus or antigens. Typically, biologics do not target metabolic pathways like antiviral drugs, but stimulate immune cells such as lymphocytes, macrophages, and/or antigen presenting cells, in an effort to drive an improved immune response to the virus. Influenza models, such as murine influenza, are convenient models to test the effects of prophylactic and therapeutic biologics. For example, Lymphocyte T-Cell Immune Modulator inhibits viral growth in the murine model of influenza.
Medical understanding of the influenza virus, it’s composition and mechanism of action, is giving hope to a longer lasting, more universal approach to inoculation.
Sources:- https://www.cdc.gov/flu/professionals/index.htm
- https://www.who.int/topics/influenza/en/
- www.niaid.nih.gov/labsandresources/resources/ceirs/Pages/default.aspx
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4047948/
- https://www.biomedcentral.com/1741-7015/10/104
- Stephen Adams (8 July 2011). "Universal flu vaccine a step closer". The Telegraph.
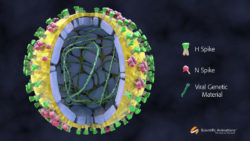
Structure of the Swine Flu Virus
Swine flu is a contagious viral infection transmitted by inhalation or ingestion of droplets containing virus from people sneezing or coughing. ... Read More..








